Opioid drugs such as morphine, oxycodone (OxyContin), hydrocodone (Vicodin), and fentanyl are the gold standard for immediate pain relief in the clinic. These drugs work by acting at the opioid receptors in the brain. Once these receptors are activated, a pain inhibition circuit is disinhibited, which acts to decrease the transmission of pain signals originating from the periphery. As a result, the sensation of pain is decreased. The action of these drugs to reduce the sensation of pain is referred to as analgesia.
However effective these drugs can be, there are risks associated with long-term opioid use. Opioid drugs may be highly addictive, as they also produce a sensation of euphoria that activates the natural dopamine reward system in the brain. According to the CDC, an estimated 46 people die each day from prescription opioid overdose.
Another side effect associated with chronic opioid use is a form of hyperalgesia. Hyperalgesia is a decreased threshold for painful stimuli. People with hyperalgesia may respond to a weak painful stimulus with a strong withdrawal response. This can also be called opioid-induced hyperalgesia (OIH) or paradoxical anesthesia.
The precise molecular mechanisms that cause opioid induced hyperalgesia are still unknown, but a few theories are being explored.
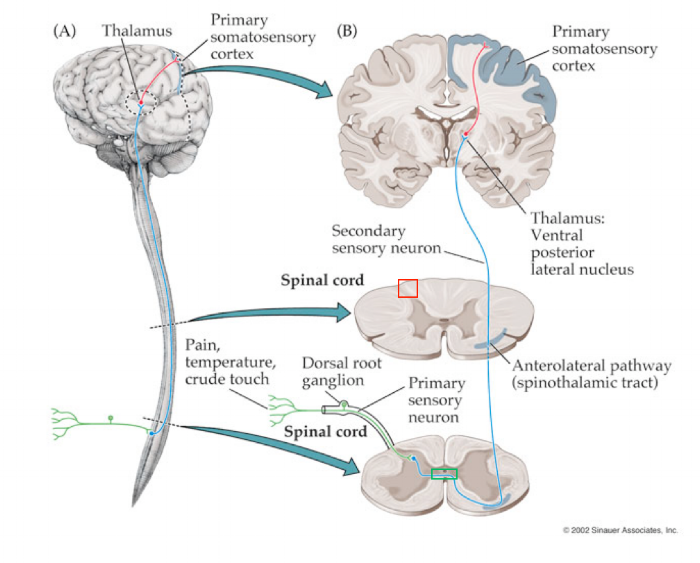
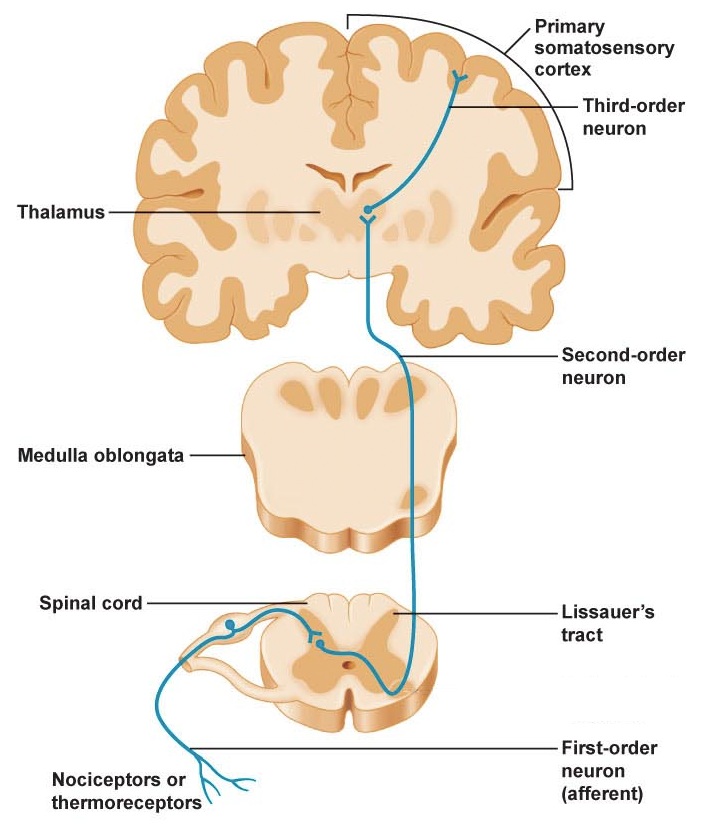
The skin is able to detect noxious stimuli in the surrounding world through activation of receptors called nociceptors. If the body experiences something that might lead to damage, these nociceptors may become active. Once active, they fire action potentials, releasing neurotransmitter at the next neuron in the spinal cord. Eventually, this pain signal passes from the spinal cord into the brain, traveling via the anterolateral tract.
The nociceptive sensitization theory holds that certain synapses in the spinal cord change after long-term opioid use. The modification is a maladaptive form of long-term potentiation (LTP). After the development of this LTP, the nociceptive input to the first neuron in the spinal cord produces a stronger signal compared to the before LTP. This results in hyperalgesia.
The hyperalgesia is believed to be associated with aberrant NMDA receptor signaling in the spinal cord. Co-administering the opioid drug with an inhibitor (antagonist) for the NMDA receptor such as ketamine has had mixed results, indicating that the disorder is more complex than believed.
Treatment of opioid-induced hyperalgesia
The hyperalgesia observed in chronic opioid treatment can be reversed. One possibility is to switch the painkiller so a different drug is used. Different drugs have slightly different pharmacological properties, meaning they have novel activity at a different class of opioid receptors. The body may then naturally reverse the maladaptive plasticity processes over the time.
The other option for treating opioid induced hyperalgesia is to reduce the dosage of the original opioid drug, but to continue to supplement treatment with a non-opioid alternative. For example, the dosage of vicodin may be decreased, but the doctor may also prescribe an acetaminophen or a Cox 2 inhibitor to take alongside the vicodin. This way, the opioid mechanisms will be activated less, and over time, the body will return to a homeostasis with less hyperalgesia. Ideally, the patient experiences the same amount of pain management since the other painkillers are present in the system.