Answer: The concentration gradient is the difference in concentration of a solute across a membrane.
The neurons of the brain allow ions to pass their cell membrane because of the presence of transmembrane proteins called ion channels. However, ions are not usually evenly distributed across the cell membrane. Some ions, like sodium (Na), chloride (Cl), and calcium (Ca2+) are very highly concentrated outside of the neurons, while potassium (K) is highly concentrated inside the neuron. Because there is a carefully regulated balance of ions across the membrane, ions do not move randomly into or out of the neuron. Instead, they follow the principles of chemistry and physics when an ion channel is open.
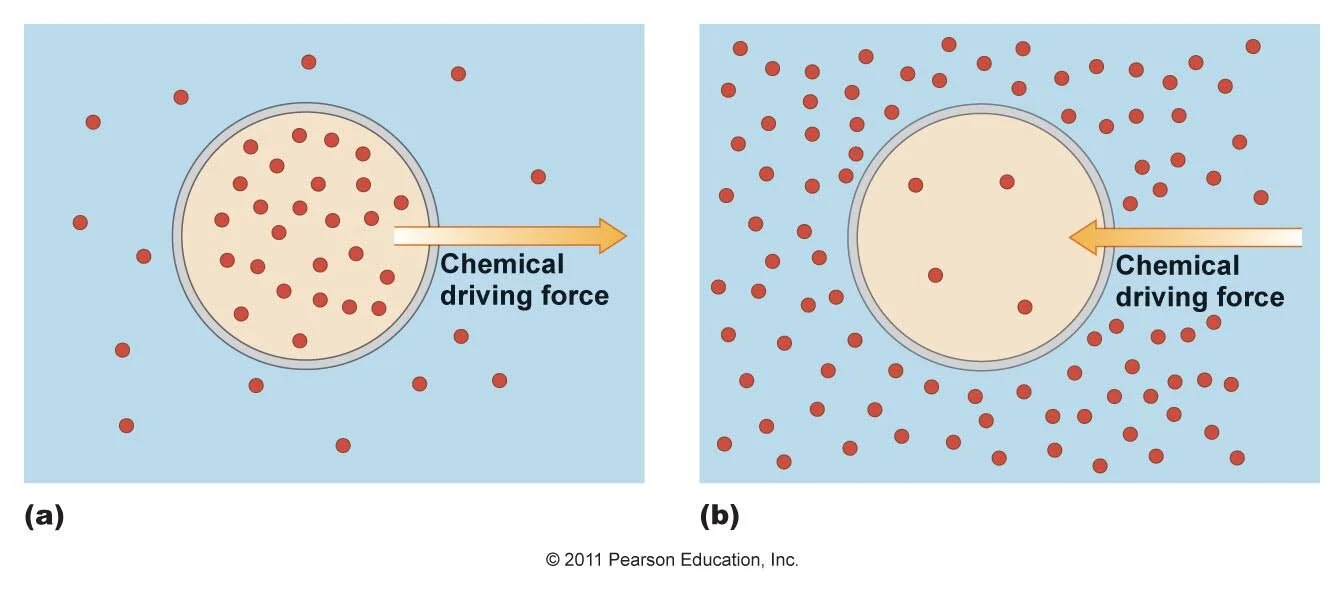
The concentration gradient refers to the difference of ion concentration inside the cell compared to outside the cell. If there is a large concentration of one ion outside the cell, the concentration gradient creates a force that will act to move ions into the neuron. Ions moving through ion channels because of this force are said to move "down their concentration gradient."
The concentration gradient is one component of the electrochemical gradient (the chemical part) that determines the direction an ion will move when a transmembrane ion channel is opened. The other component is the electrical gradient is determined by the number and quantity of electrical charges present across the membrane.
Knowing the electrochemical gradient allows us to predict which direction an ion will move across a semipermeable membrane. For example, consider a simple water-filled chamber that has been divided into two parts, separated by a membrane that allows water and sodium ions to permeate. One chamber is filled with a high concentration of sodium ions dissolved in water, while the other chamber is filled with a low concentration of sodium ions in water. Under these conditions, there is a strong chemical gradient that pushes the sodium ions from the high concentration side toward the low concentration side. This force continues to act until the concentration of sodium is equal on both sides of the semipermeable membrane, at which point there is no longer any concentration gradient. At this point, movement of ions across the semipermeable membrane is at equilibrium.
More relevant to the brain, the electrochemical gradient gives us knowledge of ion movement during the opening or closing of an ion channel. The additional consideration in the case of biological membranes is that neurons also carry an electrical charge, and these electrical charges contribute to the forces acting on each ion.